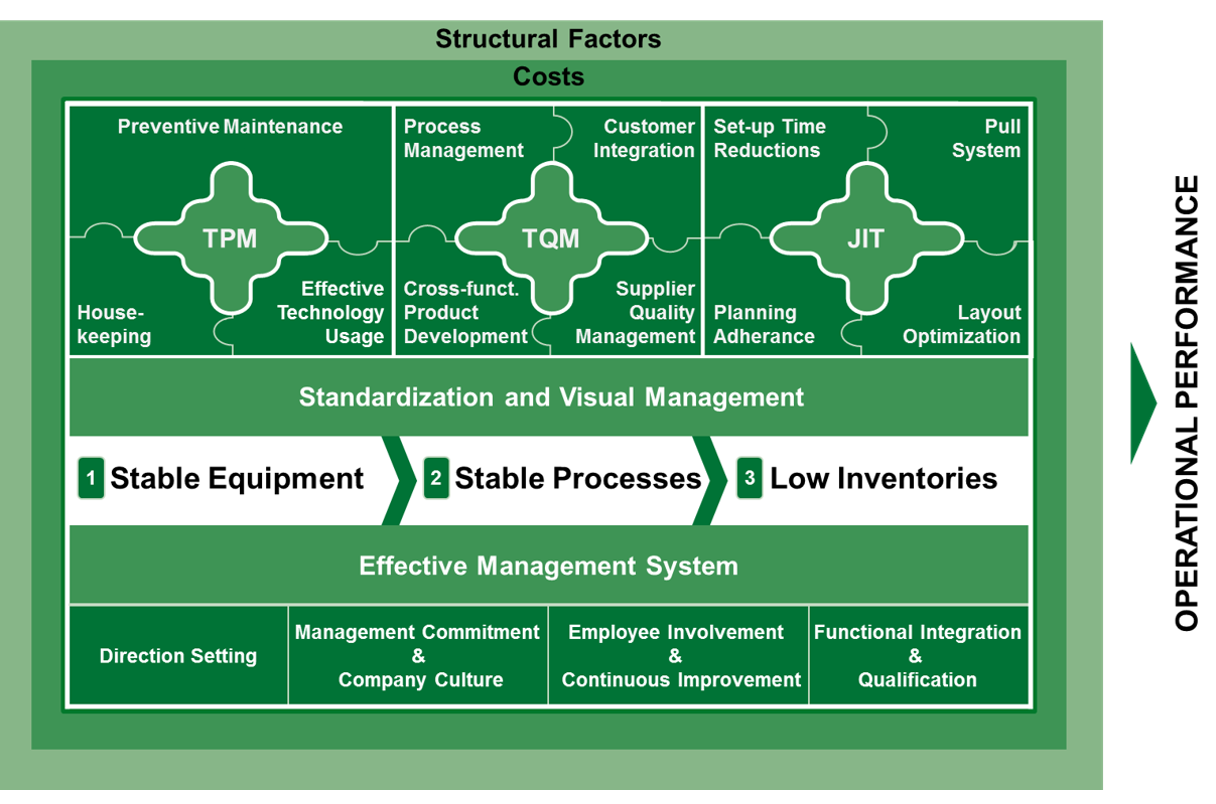
Our Operational Excellence Model
“The right implementation of basic principles of Operational Excellence will lead
to a significantly higher performance among pharmaceutical manufacturing plants
and thus to a higher overall business performance of the company."
This hypothesis was already proven by OPEX benchmarking and a number of our industry projects and is examined more profoundly using a scientific model. The model consists of the elements Total Productive Maintenance (TPM), Total Quality Management (TQM), Just-in-Time (JIT) and the underlying Management System.
Our benchmarking is based on the belief that performance (KPIs) cannot be evaluated without the consideration of the specific site environment, the structural factors, the management system or specific site roles. Consequently, our analysis sets the focus on the link between the performance scores and the enablers leading to this performance.
Research Questions
What does it need to be a Top Performer in terms of Operational Excellence?
What has changed compared to the former benchmarkings?
What is the future role of manufacturing in the pharmaceutical industry?
What are the implications for pharmaceutical manufacturing?
How should pharmaceutical manufacturing respond to the changing environment?